
Authors: O'Shannessy DJ, Ekberg B, Andersson LI, Mosbach K
Article Title: Recent advances in the preparation and use of molecularly imprinted polymers for enantiomeric resolution of amino-acid derivatives.
Publication date: 1989
Journal: Journal of Chromatography
Volume: 470
Issue: (2)
Page numbers: 391-399.
DOI: 10.1016/S0021-9673(01)83567-6
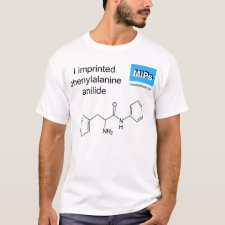
Abstract: Acrylate-based molecular imprints were prepared, using L-phenylalanine anilide as the print molecule and methacrylic acid as the functional monomer, which is believed to interact both ionically and through hydrogen bonding with the print molecule. Several aspects of the polymer preparation were investigated, including the solvent of polymerization, the initiation system and the effect of the molar ratio of functional monomer to print molecule on the ability of the polymers to separate the enantiomers of the print molecule. Polymers were analysed by high-performance liquid chromatography where the effect of particle size and eluent composition were investigated for enantiomeric resolution of the print molecule. The ability of the polymers to separate racemic mixtures of amide derivatives of amino acids other than phenylalanine anilide (print molecule) was also investigated. It was thus shown that efficient enantiomeric resolution of amide derivatives of amino acids including the anilides, p-nitroanilides, b-naphthylamides and amides of amino acids, ranging from alanine to trytophan, was possible on a single polymer imprinted with -phenylalanine anilide. Such separations were highly specific and dependent on the presence of both the print molecule and functional monomer in the polymerization mixture. The mechanism of recognition was shown to involve ionic bonding to the primary amine and hydrogen bonding to the amide function of the substate by acid groups of the polymer, "immobilized" in a stereospecific manner. Both interactions were necessary for efficient enantiometric resolution. Molecular imprints, prepared from -phenylalanine anilide, were also shown to give efficient enantiomeric resolution of phenylalanine-containing dipeptides and may be useful for preparative-scale separations.
Template and target information: L-phenylalanine anilide

Join the Society for Molecular Imprinting

New items RSS feed
Sign-up for e-mail updates:
Choose between receiving an occasional newsletter or more frequent e-mail alerts.
Click here to go to the sign-up page.
Is your name elemental or peptidic? Enter your name and find out by clicking either of the buttons below!
Other products you may like:
 MIPdatabase
MIPdatabase









